Entri yang Diunggulkan
- Get link
- X
- Other Apps
Staff exposure to EtO. Throughout the facility where sterile processing occurs.
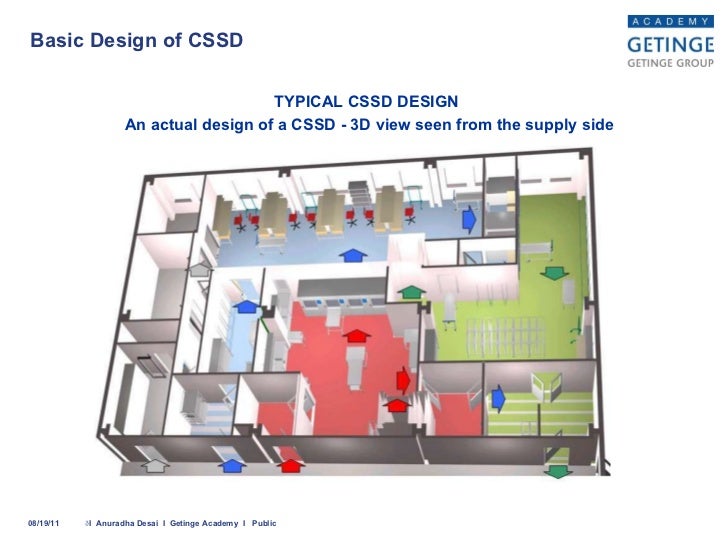
 Fgi Requirements For Sterile Processing Facilities Health Facilities Management
Fgi Requirements For Sterile Processing Facilities Health Facilities Management
4009 - Sterile Supplies in Surgical Services Department.

Central sterile supply department guidelines. 4014 - Disposal of Sharps. Department CPD central supply central service central services sterile processing and distribution SPD etc. 4013 - Hand Hygiene - CDC Guidelines.
Employee must be trained in a safe work procedure and be aware of any relevant procedures policies. The work flow for the central sterile supply department is centered on the processing of soiled instruments through the four zones. 4007 - Stocking Responsibilities.
Three different types of sterilization are used in CSD. Central sterilesterile processing department CSSPD professionals have access to a number of industry standards and guidelines that offer recommendations and best practices for the effective and safe processing of instruments from organizations such as the American National Standards Institute ANSI Association for the Advancement of Medical Instrumentation AAMI Association of. 2Clean for dirty exchange.
4015 - Sharps Injury Protection Plan. Each department set predetermined stock level for each user and distributing routinely with record. The terms sterile processing central services and central supply are the more commonly used terms for the department today.
Demand for box trolley basket containing article for need for specific period. 4011 - Standard Precautions. EtO is used within central supply as a sterilant for items that can not be exposed to steam sterilization.
Procedure General Guidelines All personnel must follow established work and traffic flow patterns. The Department expects that general hospitals and diagnostic and treatment centers authorized to provide ambulatory surgical services which have central service departments that perform decontamination preparation packaging sterilization and storage and distribution of reusable medical instrumentation or devices and employ CSTs will be impacted by this law and will need to develop. A distinct separation must be maintained between the soiled and sterile areas.
4006 - Cleaning Central Service Department. 4012 - Aseptic Practices. 819 The floors and walls should be constructed of materials capable of withstanding chemical agents used for cleaning or disinfecting.
The current OSHA Permissible Exposure Limit PEL for EtO is 1 ppm for an 8hr time weighted average with a 5ppm excursion level. Procedures should include maintaining records of monitoring results that are retrievable either from a central. 4010 - Stock Rotation.
The sterilization department must be able to provide the operating theatre and wards with a. One clean article for each dirty is exchanged 3Regular complete stock issue. It is suitable for instruments that can withstand high temperature and humidity.
The technical staff works on either the soiled side or the sterile side and cannot cross from one side to the other. The sterile storage area should be a limited access area with a controlled temperature may be as high as 75F and relative humidity 30-60 in all works areas except sterile storage where the relative humidity should not exceed 70. Basic guidelines for disinfection and sterilization 8.
Items to be sterilized are subject to specific method of sterilization as per the manufacturers guidelines. Even the smallest health facility will need sterile surgical instruments for minor surgical procedures and sterile dressing material. Material Safety Data Sheets MSDS for all chemicals used in the sterile service department must be available in a binder index.
The operation of this service shall be carried out in. 4008 - Storage of Sterile Supplies. Central Sterile Supply a Each hospital shall provide prepare sterilize and store sufficient sterile supplies and medical and surgical equipment and shall dispense them to all services in the hospital.
4 With the centralization of the pre-disinfection cleaning packing and sterilization of all items in one department it is of paramount importance to provide consistently high standards in the sterilization techniques and product quality. Steam Sterilization Pre-vacuum cycle is used. Ceilings and wall surfaces.
Distribution from Sterile store Four major system of distribution 1Topping up. 4Ordinary order system straight. Microsoft PowerPoint - guidlinesenglish Author.
Historically more to come on this topic in the following historical module the department was born. Establish policies and procedures for monitoring and maintaining HVAC parameters within the sterile processing areas. The Central Sterile Supply Department is responsible for preparing medical surgical supplies and equipment so that they are sterile and ready for use in patient care.
Every hospital must have the means to sterilize equipment and supplies. Sterilization 10Correctly loading the sterilizer 11Handling transporting and storing materials 12Methods for controlling the sterilization process 13Failures in the sterilization process 14Validating the.
 Central Sterile Services Department Planning Designing
Central Sterile Services Department Planning Designing
What Is Cssd Or Sterile Processing Department Cssd Technician Hub
Sterile Processing Guidelines And Objectives Cssd Technician Hub
 Central Sterile Supply Department
Central Sterile Supply Department
 Basics Of Central Sterile Supply Orientation Sterileprocessing Department
Basics Of Central Sterile Supply Orientation Sterileprocessing Department
 3 Tips For An Effective Central Sterile Supply Department
3 Tips For An Effective Central Sterile Supply Department
Http Www Icdkwt Com Pdf Policiesandguidelines Decontamination Guidelines English Pdf
Http Www Icdkwt Com Pdf Policiesandguidelines Decontamination Guidelines English Pdf
 Central Sterile Services Department Planning Designing
Central Sterile Services Department Planning Designing
 Central Sterile Supply Department
Central Sterile Supply Department
 Pdf Role Responsibility Of Central Sterile Supply Department Sterile Supply Department In Hic
Pdf Role Responsibility Of Central Sterile Supply Department Sterile Supply Department In Hic
Central Sterile Services Working Space
 Central Sterile Supply Department Cssd Patient Safety Starts Here
Central Sterile Supply Department Cssd Patient Safety Starts Here
Comments
Post a Comment