Entri yang Diunggulkan
- Get link
- X
- Other Apps
On December 7 2016 the Office of Inspector General the OIG of the US. The Anti-Kickback Statute codified at 42 US.
 Anti Kickback Statute Stark Ekra Whistleblower Law
Anti Kickback Statute Stark Ekra Whistleblower Law
The Federal Anti-Kickback Statute Promotional Discount Programs Discounts and Rebates FAQ Does a dental practices participation in a federal healthcare program such as Medicaid TRICARE or Medicare Advantage affect its ability to participate in a discount or rebate program with a.

The anti kickback statute. In return for referring an individual to a person for the furnishing or arranging for the furnishing of any item or service for which payment may be made in whole or. Anti-kickback Statute provides that any person who knowingly and willfully solicits receives oflers or pays remuneration directly indirectly overtly or covertly for. The federal Anti-Kickback Statute AKS is one of the best-known federal fraud and abuse statutes due largely to its wide-ranging effects on business relationships in the health care pharmaceutical and medical device sectors.
While providers have had the option of using flexible waivers of the Stark Law and Anti-Kickback Statute under the Medicare. The Anti-Kickback Statute is broadly drafted and prohibits both the receipt and the offering to pay or payment of the kickback. The federal Anti-Kickback Statute AKS See 42 USC.
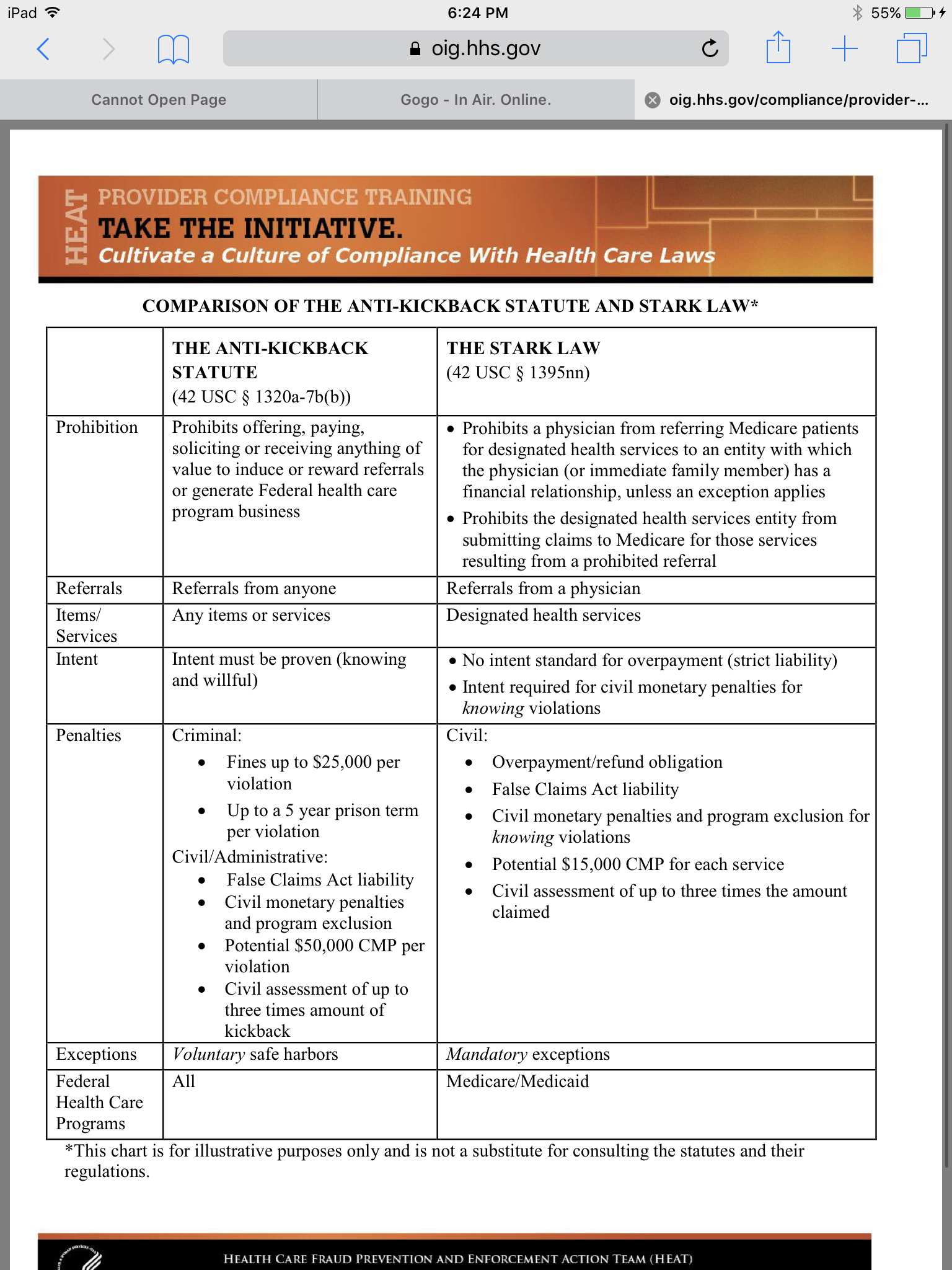
As health care providers transition to value-based care models they have often been forced to rely on exceptions and safe harbors under the Stark Law and Anti-Kickback Statute AKS that were never designed with value-based payment arrangements in mind. COMPARISON OF THE ANTI-KICKBACK STATUTE AND STARK LAW THE ANTI-KICKBACK STATUTE 42 USC 1320a-7bb THE STARK LAW 42 USC 1395nn Prohibition Prohibits offering paying soliciting or receiving anything of value to induce or reward referrals or generate financial relationship unless an exception appliesFederal health care. The AKS is a criminal statute that prohibits transactions intended to induce or reward referrals for items or services reimbursed by the federal health care programs.
On November 20 2020 the Department of Health and Human Services HHS Office of Inspector General OIG issued a final rule Revisions to Safe Harbors under the Anti-Kickback Statute and Civil Monetary Penalties Rules. Code 1320a7b b is an American federal law which imposes criminal and particularly in association with the federal False Claims Act civil liability on those that knowingly and willfully offer solicit receive or pay any form of remuneration in exchange for the referral of services or products covered by any federal healthcare program eg the referral of a. Jazz Pharmaceuticals Lundbeck and Alexion Pharmaceuticals to settle kickback allegations.
1320a-7b is a criminal statute that prohibits the exchange or offer to exchange of anything of value in an effort to induce or reward the referral of business reimbursable by federal health care programs. The Anti-Kickback Statute restricts a pharmaceutical firm from offering or paying directly or indirectly any remuneration in the form of money or any other thing of value to motivate Medicare or ChampVA patients to purchase the companys drugs. The Stark Law and Anti-Kickback Statute are the main anti-fraud tools targeting the health-care industry and are designed to keep caregivers personal financial considerations from influencing their medical decisions.
Congress originally enacted the Anti-Kickback Statute as part of the Social Security Amendments of 1972. To prove a violation of the Anti-Kickback Statute the government need only show that a health care provider willfully gave or received something of value in exchange for the referral of a MedicareMedicaid patient. Department of Health and Human Services HHS issued a final rule modifying the safe harbors to the anti-kickback statute 1 and the civil monetary penalties CMP rules 2 as well as a policy statement adjusting the monetary value of nominal gifts not subject to the CMP prohibition against beneficiary inducements.
A Brief Summary of the Stark Law and Anti-Kickback Statute Reforms Final Rules Background. HHS Finalizes New Protections for Value-Based Arrangements and Other Revisions to the Anti-Kickback Statute Safe Harbors and Civil Monetary Penalties Exceptions. On December 2 2020 the Department of Health and Human Services HHS Office of Inspector General OIG published the final rule Revisions to the Safe Harbors Under the Anti-Kickback Statute AKS.
On January 19 2021 the final rule updating the safe harbors under the federal Anti-Kickback Statute AKS and adding a new exception to the Beneficiary Inducements Civil Monetary Penalties CMP takes effect. Until then only one provision sanctioned false.
 The Anti Kickback Statute An Educational Video
The Anti Kickback Statute An Educational Video
 Do The Anti Kickback And Stark Laws Apply To Private Payors Medicaidlaw Nc
Do The Anti Kickback And Stark Laws Apply To Private Payors Medicaidlaw Nc
 The Anti Kickback Statute What Constitutes A Referral Florida Healthcare Law Firm Blog
The Anti Kickback Statute What Constitutes A Referral Florida Healthcare Law Firm Blog
 Changes To Federal Laws May Mean Better Collaboration Between Healthcare Providers Enttoday
Changes To Federal Laws May Mean Better Collaboration Between Healthcare Providers Enttoday
 Anti Kickback Statute Kickbacks In Healthcare Phillips Cohen
Anti Kickback Statute Kickbacks In Healthcare Phillips Cohen
 The Stark Law And Anti Kickback Statute What To Expect Erdman
The Stark Law And Anti Kickback Statute What To Expect Erdman
 Is A Violation Of The Anti Kickback Law Also A Violation Of The False Claims Act Zuckerman Law
Is A Violation Of The Anti Kickback Law Also A Violation Of The False Claims Act Zuckerman Law
 There S A New Anti Kickback Statute That Most Practices Don T Know About
There S A New Anti Kickback Statute That Most Practices Don T Know About
 Anti Kickback Lawyers Goldberg Kohn
Anti Kickback Lawyers Goldberg Kohn
 Anti Kickback Statute Vs Stark Law Qui Tam Lawyers Berger Montague
Anti Kickback Statute Vs Stark Law Qui Tam Lawyers Berger Montague
 Amazon Fr What Is The Anti Kickback Statute Crane Thomas S Kingsbury Samantha Lovitch Karen Roll Carrie American Bar Association Livres
Amazon Fr What Is The Anti Kickback Statute Crane Thomas S Kingsbury Samantha Lovitch Karen Roll Carrie American Bar Association Livres
![]() Stark Law And Anti Kickback Statues Basics
Stark Law And Anti Kickback Statues Basics
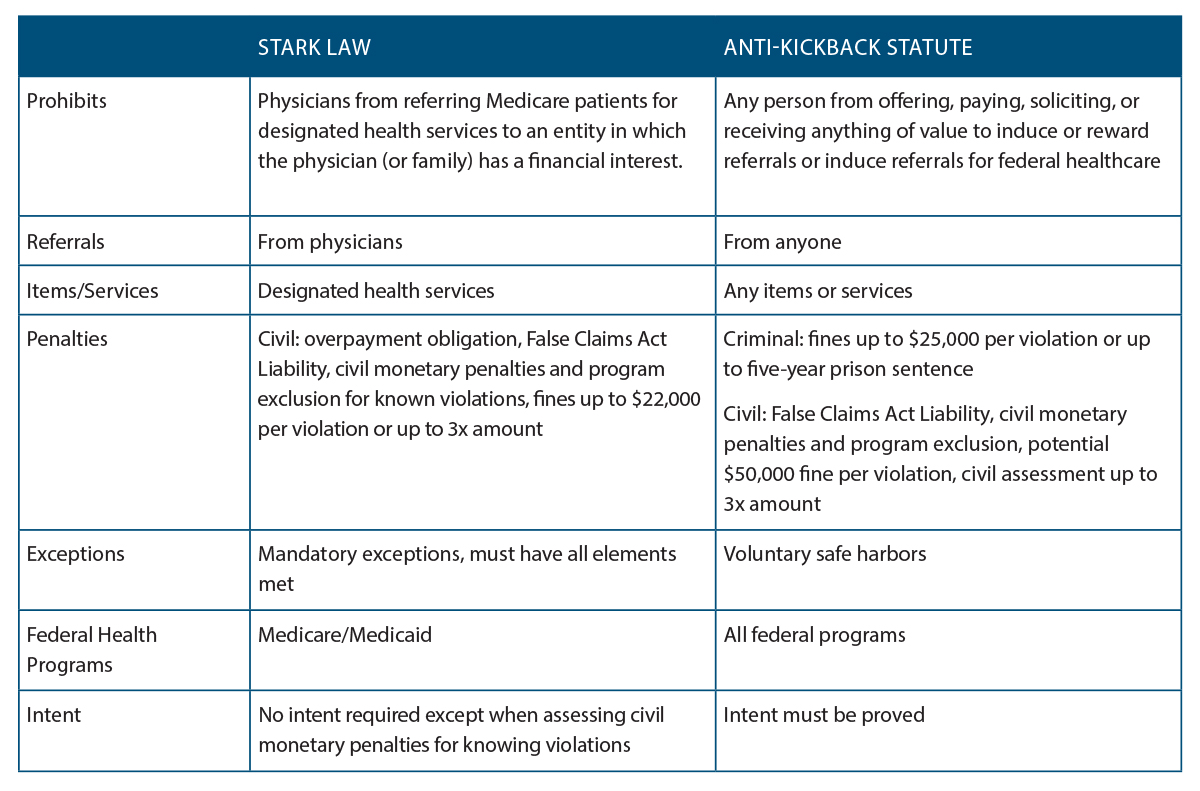
 The Anti Kickback Statute Stark Law Key Differences Examples
The Anti Kickback Statute Stark Law Key Differences Examples
Anti Kickback Statute Explained Houston Health Care Fraud Defense Attorneys
Comments
Post a Comment