Entri yang Diunggulkan
- Get link
- X
- Other Apps
DUBLIN March 30 2021 PRNewswire -- Jazz Pharmaceuticals plc Nasdaq. 3 2017 PRNewswire -- Jazz Pharmaceuticals plc Nasdaq.
 Rx Item Vyxeos Daunorubicin And Cytarabine Liposome Injection Powder By Jaz
Rx Item Vyxeos Daunorubicin And Cytarabine Liposome Injection Powder By Jaz
Jazz Pharmaceuticals is committed to helping healthcare professionals provide appropriate care for their patients.

Vyxeos jazz pharmaceuticals. Personal Information All fields are required. Um das Risiko einer Gewebsnekrose zu vermeiden sollte darauf geachtet werden dass keine Paravasate. Vyxeos - Jazz Pharmaceuticals Reference number DACPM2021-21 II12 Main CPV code 33000000 - Medical equipments pharmaceuticals and personal care products II13 Type of contract Supplies II14 Short description Vyxeos - Jazz Pharmaceuticals II16 Information about lots This contract is divided into lots.
Vyxeos is the first new chemotherapy advance in more than 40 years for adults with newly-diagnosed therapy-related AML or AML with myelodysplasia-related changes said Bruce Cozadd chairman and chief executive officer of Jazz Pharmaceuticals. Food and Drug Administration FDA approved a revised label for Vyxeos daunorubicin and cytarabine to include a new indication to treat newly-diagnosed therapy-related acute myeloid leukemia t-AML or AML with myelodysplasia-related. Jazz Pharmaceuticals is a global biopharmaceutical company dedicated to bringing life-changing medicines to people with limited or no options so they can live their lives more fully.
Vyxeos liposomal ist ausschließlich zur intravenösen Anwendung bestimmt. Dawkowanie produktu Vyxeos liposomal jest inne niż dawkowanie daunorubicyny i cytarabiny podawanych we wstrzyknięciu dlatego nie wolno go stosować zamiennie z innymi produktami zawierającymi daunorubicynę i lub cytarabinę patrz punkt 44. The information on the website you are entering is intended for visitors in the United.
Vyxeos liposomal wird als intravenöse Infusion über einen Zeitraum von 90 Minuten angewendet. Nie wszystkie wielkości opakowań muszą znajdować się w obrocie. Es darf nicht intramuskulär intrathekal oder subkutan angewendet werden.
Please provide us with the above information so that we or third parties working on our behalf can send you information services and materials about VYXEOS or. And markets Erwinase Defitelio defibrotide and Vyxeos 44 mg100 mg powder for concentrate for solution for. Jazz Pharmaceuticals markets Xyrem sodium oxybate oral solution Erwinaze asparaginase Erwinia chrysanthemi Defitelio defibrotide sodium and Vyxeos daunorubicin and cytarabine liposome for injection in the US.
By clicking on this page you confirm you are a US. Thank you for visiting. Jazz Pharmaceuticals Europe and Rest of World senior vice-president Iain McGill said.
Savings CardIf you are eligible and commercially insured you may pay as little as 10 per prescription subject to an annual maximum. VYXEOS is supplied as a single-dose vial and does not contain any preservatives. You are leaving the Vyxeos-4 page.
As Celator is currently preparing a regulatory submission in the US. Follow applicable special handling and disposal procedures. Reconstitute and further dilute VYXEOS prior to intravenous infusion.
Dawkowanie Dawka produktu Vyxeos liposomal zależna jest od powierzchni ciała ang. For Vyxeos daunorubicin and. Jazz Pharmaceuticals reserves the right to terminate or modify this program at any time with or without notice.
Do not save any unused portions for later administration. Click Cancel to return or OK to continue. JAZZ today announced that the US.
Thank you for contacting Jazz Pharmaceuticals Corporate Development Partnering. Jazz Pharmaceuticals Announces FDA Approval of Additional Indication for Vyxeos daunorubicin and cytarabine for the March. This page is for US.
Food and Drug Administration FDA has approved Vyxeos daunorubicin and cytarabine. Każde opakowanie zawiera 1 fiolkę 2 fiolki lub 5 fiolek. Celator Pharmaceuticals is a strong strategic fit with Jazz Pharmaceuticals.
JazzCares is committed to helping you reduce your out-of-pocket costs for VYXEOS. Jazz is delighted that Vyxeos will now be made routinely available on the NHS in England and Wales as people with therapy-related AML or AML with myelodysplasia-related changes have had limited treatment options until now. JAZZ today announced that the US.
Jazz Pharmaceuticals is not responsible for the content or privacy practices of any third-party websites. Lek Vyxeos liposomal to fioletowy proszek do sporządzania koncentratu roztworu do infuzji dostępny w szklanej fiolce. VYXEOS will further diversify our product portfolio and is complementary to our clinical and commercial expertise in hematologyoncology said Bruce Cozadd chairman and chief executive officer of Jazz Pharmaceuticals plc.
VYXEOS is a cytotoxic drug. Podmiot odpowiedzialny i wytwórca Jazz Pharmaceuticals Ireland Ltd 5th Floor Waterloo Exchange. Contact Corporate Development Partnering.
On August 3 2017 the FDA approved Jazz Pharmaceuticals Vyxeos as a treatment option for adults suffering from either newly diagnosed therapy-related acute myeloid leukemia or t-AML or from.
 Jazz Pharmaceuticals Vyxeos Granted Accelerated Assessment Contract Pharma
Jazz Pharmaceuticals Vyxeos Granted Accelerated Assessment Contract Pharma
Social Media Monitoring On Vyxeos Research And Study On Social Media Channels
Https Assets Publishing Service Gov Uk Media 5c936d7040f0b633fbec81fc Vyxeos Dhpc Pdf
 Jazz Pharmaceuticals Seeks Fda Drug Application Approval For Vyxeos
Jazz Pharmaceuticals Seeks Fda Drug Application Approval For Vyxeos
 Fda Approves Vyxeos For Secondary Acute Myeloid Leukemia In Pediatric Patients
Fda Approves Vyxeos For Secondary Acute Myeloid Leukemia In Pediatric Patients
 Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Vyxeos Chemotherapy Liposome Injection For Acute Myeloid Leukemia Ppt Download
Https Investor Jazzpharma Com Static Files 17ce025e Ffe8 4ffb 9eb0 1b557615735b
Jazz Pharma S Vyxeos Secures Nice Recommendation In Acute Myeloid Leukaemia Pharmafile
 Vyxeos Jazz Pharmaceuticals Inc Fda Package Insert
Vyxeos Jazz Pharmaceuticals Inc Fda Package Insert
 Jazz Pharmaceuticals Submits Vyxeos Marketing Authorization Application To European Medicines Agency For Treatment Of Certain Types Of High Risk Acute Myeloid Leukemia
Jazz Pharmaceuticals Submits Vyxeos Marketing Authorization Application To European Medicines Agency For Treatment Of Certain Types Of High Risk Acute Myeloid Leukemia
 Jazz Pharmaceuticals Gets Fda Nod For New Indication Of Vyxeos Drug Store News
Jazz Pharmaceuticals Gets Fda Nod For New Indication Of Vyxeos Drug Store News
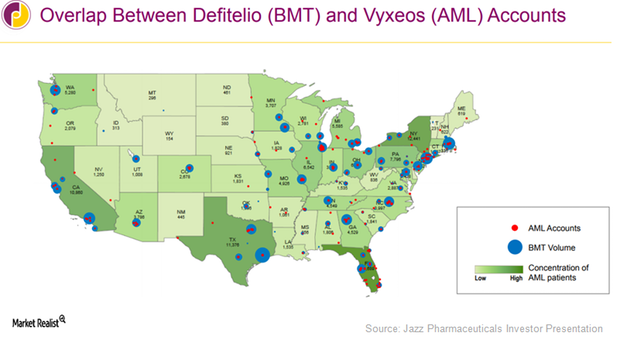 How Jazz Pharmaceuticals Aims To Boost Vyxeos Sales In 2017
How Jazz Pharmaceuticals Aims To Boost Vyxeos Sales In 2017


Comments
Post a Comment