Entri yang Diunggulkan
- Get link
- X
- Other Apps
When they prioritize their profits over the people to whom they sell their products someone needs to hold them accountable. Easily research and order everything your lab needs.
 Waters Uplc And Mass Spectrometry Systems Approved For In Vitro Diagnostic Use In Brazil Medical Product Outsourcing
Waters Uplc And Mass Spectrometry Systems Approved For In Vitro Diagnostic Use In Brazil Medical Product Outsourcing
Waters follows internationally recognized standards with respect to its medical devices.

Waters medical devices. Robedt Warren General Manager Waters Medical Systems LLC 2112 -l5th Street NW F EB 1 6 2012 ROCHESTER MN 55901 Re. Keeping pace with medical device innovation The availability of modern polymer analysis. Join the discussion and hear first-hand how industry experts have built effective and compliant risk-management workflows that help smooth the path of medical device product to market.
Waters COVID Innovation Symposium Contain the Strain. At Walters Medical we address everything we believe is important in a medical supply company. This ever-expanding and versatile system portfolio provides options as you implement LC-MSMS for routine use in the clinical laboratory.
Watch our on-demand medical devices testing webinar series to learn more about the critical nature of chemical characterization mechanical testing fatigue and durability assessment and rheological study of soft biomaterials. Benefit from Waters patented sub-2-μm hybrid particle chemistry. In the United States the design manufacture and servicing of medical devices are governed by 21 CFR Part 820 Quality System.
From adhesive bandages to heart valves medical devices are used in the diagnosis prevention monitoring and treatment of a broad array of medical conditions. While this framework is intended to safeguard patient safety the regulations and standards that support them have historically been surprisingly outdated and fragmented. Waters is the leading provider of lab equipment supplies and software for scientists across the world.
ISO10993 defines extractables as the potential impact a substance that is released from a medical device or material of construction when the medical device or material is extracted using laboratory extraction conditions and vehicles. DEPARTMENT OF HEALTH HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Room -W066-G609 Silver Spring NID 20993-0002 Mr. Medical devices are essential for the health and well-being of millions of patients worldwide.
Research Use Only RUO instruments and reagents are not intended for clinical diagnostic use. Waters manufactures and supports an expanding portfolio of medical devices for in vitro diagnostic use that are designed to offer clinical laboratories access to the benefits of LC and MS technologies. This ever-expanding and versatile system portfolio provides options as you implement LC-MSMS for.
Clinical laboratories now have access to the selectivity sensitivity and versatility of LC-MSMS analysis not seen using traditional techniques. K1 11521 TradeDevice Name. Our aim is to deliver a unique combination of specialist critical care products service provision and.
Our kidney perfusion system provides secured environment where a physiologic solution is pumped through the explanted organs to minimize tissue damage. Whether increasing efficacy and reliability reducing manufacturing costs or delivering the highly personalized devices of the future the medical devices industry depends on materials science to deliver products that are fit for their intended use and that also meet both regulatory and performance requirements. Waters Corporation manufactures IVD medical devices that offer clinical diagnostic laboratories an alternative to LC-MSMS systems manufactured for clinical research use only.
Waters medical devices are designed to optimize your laboratorys workflow and span from pre-analytics prep solutions to analytics LC to LC-MSMS to post-analytics informatics. A dedicated range of quality products at realistic prices great customer service and a can do attitude. The timeline for bringing a medical device to market can average anywhere between 3 to 7 years and requires manufacturers to navigate through a complex regulatory framework.
We provide machine perfusion and static preservation techniques specific to organ types and adapted to transplant teams preferences. We are proud to take on these large companies to advocate for the consumers. Waters medical devices are designed to optimize your laboratorys workflow and span from pre-analytics prep solutions to analytics LC to LC-MSMS to post-analytics informatics.
Would you like to discuss one of the topics below with a Waters Medical Devices expert. Advancing an Innovation Ecosystem for Infectious Disease Preparedness 801 2020 New Englander of the Year Awards Celebration Udit Batra Acceptance Speech. Watch our on-demand medical devices testing webinar series to gain expert insight into the critical factors involved in medical device design testing and regulatory compliance including mechanical fatigue and durability testing and the rheological study of soft biomaterials.
Extractables and leachables testing is intended to identify and quantify harmful species which could leach from medical devices and pose a health risk to users. Please choose one Chemical Characterization of Medical Devices Mechanics and Strength for Biomaterials Fatigue and Durability of Medical Devices Rheology of soft Biomaterials Microcalorimetry Testing of Medical Devices Thermal Analysis of Medical Devices Other topics I dont want to be contacted. Medical device manufacturers have a duty to represent to you the known dangers of their products.
Evolving regulations are dramatically changing the medical device industry. Please complete the form below to receive your complimentary copy of our Medical Device Regulatory Pathway eBook.
 Lc Ms Clinical Diagnostics Solutions Waters
Lc Ms Clinical Diagnostics Solutions Waters
 Ivd Medical Device Systems Infographic
Ivd Medical Device Systems Infographic

 Lc Ms Clinical Diagnostics Solutions Waters
Lc Ms Clinical Diagnostics Solutions Waters
 A Lifeport Kidney Transporter Organ Recovery Systems B Rm3 Download Scientific Diagram
A Lifeport Kidney Transporter Organ Recovery Systems B Rm3 Download Scientific Diagram
 Uplc Uhplc Mass Spectrometry System Available For Use As A Medical Device In The Clinical Laboratory Waters
Uplc Uhplc Mass Spectrometry System Available For Use As A Medical Device In The Clinical Laboratory Waters
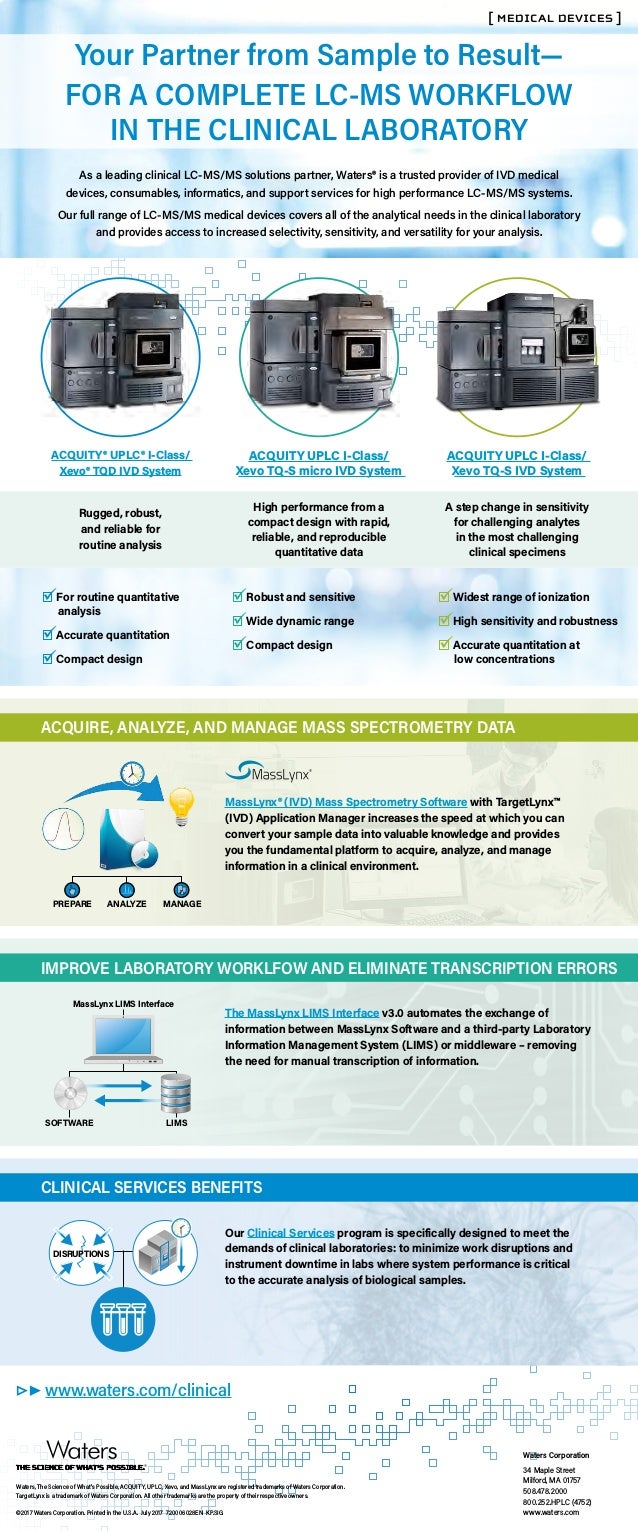 Ivd Medical Device Systems Infographic
Ivd Medical Device Systems Infographic
 Medical Devices Safety Compliance And Characterization
Medical Devices Safety Compliance And Characterization
Waters Corporation The Science Of What S Possible
 Lc Ms Ivd Labeled Medical Devices For The Clinical Laboratory Waters
Lc Ms Ivd Labeled Medical Devices For The Clinical Laboratory Waters
 Waters Medical Devices Regulatory Pathway
Waters Medical Devices Regulatory Pathway
 Lc Ms Clinical Diagnostics Solutions Waters
Lc Ms Clinical Diagnostics Solutions Waters
 Waters Manufactures General Use Ivd Medical Devices With Optimum Performance And Reliability Waters Videos
Waters Manufactures General Use Ivd Medical Devices With Optimum Performance And Reliability Waters Videos

Comments
Post a Comment