Entri yang Diunggulkan
- Get link
- X
- Other Apps
Adakveo is approved to help reduce frequency of VOCs among members aged 16 years and older with sickle cell disease and prior vasooclusive crises. States just weeks after winning US.
 Treating Sickle Cell Anemia Science
Treating Sickle Cell Anemia Science
Food and Drug Administration FDA approved the sickle cell disease medication Voxelotor for market on 25 November fully 3 months ahead of a statutory deadline for agency action.
New sickle cell medication. The drug HydroxyUrea officially approved for the treatment of the disease in 1998 will provide the best relief for sickle cell patients in the. The new drug Oxbryta attacks the underlying causes of sickle cell anemia not just the symptoms. 18 2019 HealthDay News -- A new drug to reduce a serious complication of sickle cell disease has been approved by the US.
Hydroxyurea which was the first FDA-approved therapeutic for SCD works in part by increasing fetal globin. Results were consistent across patient subgroups despite differences in disease severity genotype or background therapy. Oxbryta treatment is approved for members 12 years of age and older with sickle cell disease and a pretreatment hemoglobin level of 105 gdL or less.
Several experimental drugs including a gene. Two New Drugs Help Relieve Sickle-Cell Disease. Adakveo and Oxbryta could be revolutionary treatments but each costs about 100000 per year and must be taken for life.
Food and Drug Administration today approved Endari L-glutamine oral powder for patients age five years and older with sickle cell disease to reduce severe complications associated with. Approval following an early campaign to. New Sickle Cell Disease Drug Approved by FDA Privacy Trust Info MONDAY Nov.
The Food and Drug Administration on Friday approved Novartis Adakveo to prevent blood-vessel blockages in patients with sickle cell anemia that can be painful and sometimes fatal. Investigational therapy crizanlizumab SEG101 formerly SelG1 approximately doubled the time to first on-treatment sickle cell pain crisis according to new subgroup analysis of Phase II SUSTAIN data. The following list of medications are in.
However this soon might change. The FDA has granted fast track approval for the drug Oxbryta. Adakveos OK comes on the heels of the 2017 approval of Emmaus Medicals Endari the first new sickle cell drug in 20 years.
You usually take it as a capsule once a day. Sickle cell disease is a lifelong illness. Food and Drug Administration approved a new drug on Friday that reduces the complications associated with sickle cell disease the first drug approved for the blood disorder in more than.
A blood and bone marrow transplant is currently the only cure for sickle cell disease but there are effective treatments that can reduce symptoms and prolong life. Your healthcare team will work with you on a treatment plan to reduce your symptoms and manage the condition. FDA Approves Crizanlizumab A New Drug For Sickle Cell Disease.
Anemia Sickle Cell drug therapy. 4 Adakveo represents the first FDA. Reuters - Novartis AG has secured Medicaid coverage for a pricey new sickle cell disease therapy in two US.
Presenting their findings to the American Chemical Society researchers are now developing a new drug that could address the root cause of sickle cell disease. I am a writer journalist professor systems modeler computational and. Food and Drug Administration.
But Who Will Pay. Antibodies Monoclonal Humanized administration dosage. Triggered by a faulty gene sickle cell disease causes haemoglobin a protein in red blood cells that carries oxygen to take on a rigid sickle-like shape.
Get more advice about living with sickle cell disease. Antibodies Monoclonal Humanized. Medicine for sickle cell pain.
Basel November 15 2019 Novartis announced today that the US Food and Drug Administration FDA approved Adakveo crizanlizumab previously known as SEG101 to reduce the frequency of vaso-occlusive crises VOCs or pain crises in adult and pediatric patients aged 16 years and older with sickle cell disease. If you continue to have episodes of pain a medicine called hydroxycarbamide hydroxyurea may be recommended. Food and Drug Administration granted accelerated approval to Oxbryta voxelotor for the treatment of sickle cell disease SCD in adults.
Opinions expressed by Forbes Contributors are their own. November 25 2019 Today the US. 64 Zeilen Drugs used to treat Anemia Sickle Cell.
Nonetheless some patients do not respond to treatment with hydroxyurea and there is.
 Nih Researchers Create New Viral Vector For Improved Gene Therapy In Sickle Cell Disease National Institutes Of Health Nih
Nih Researchers Create New Viral Vector For Improved Gene Therapy In Sickle Cell Disease National Institutes Of Health Nih
 New Tablet Strength Of Sickle Cell Anemia Treatment Siklos Available Mpr
New Tablet Strength Of Sickle Cell Anemia Treatment Siklos Available Mpr
 Sickle Cell Anemia Patients May Benefit From Diabetes Drug Metformin Study Suggests
Sickle Cell Anemia Patients May Benefit From Diabetes Drug Metformin Study Suggests
Novartis Sickle Cell Medicine Adakveo Approved In Europe To Prevent Recurrent Vaso Occlusive Crises Novartis
 New Tool Measures Whether Kids With Sickle Cell Are Taking Meds
New Tool Measures Whether Kids With Sickle Cell Are Taking Meds
 New Sickle Cell Drugs From Novartis Gbt Need Big Discounts Icer Draft Fiercepharma
New Sickle Cell Drugs From Novartis Gbt Need Big Discounts Icer Draft Fiercepharma
 Efficacy And Safety Of Recently Approved Drugs For Sickle Cell Disease A Review Of Clinical Trials Experimental Hematology
Efficacy And Safety Of Recently Approved Drugs For Sickle Cell Disease A Review Of Clinical Trials Experimental Hematology
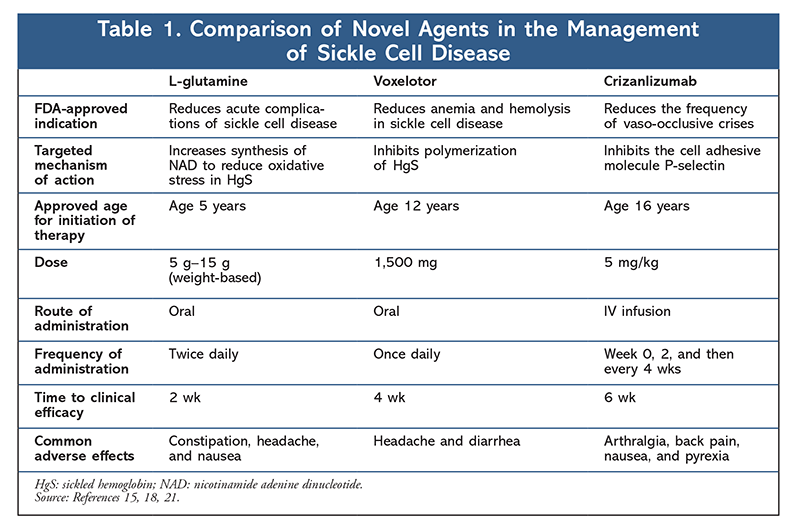 Advances In The Management Of Sickle Cell Disease
Advances In The Management Of Sickle Cell Disease
 10 Myths And Fact About Sickle Cell Disease Sickle Cell Intervention
10 Myths And Fact About Sickle Cell Disease Sickle Cell Intervention
 Novartis Eyes 1b Sales With Fda Nod For Targeted Sickle Cell Disease Drug Adakveo Fiercepharma
Novartis Eyes 1b Sales With Fda Nod For Targeted Sickle Cell Disease Drug Adakveo Fiercepharma
:max_bytes(150000):strip_icc()/nutrition-in-sickle-cell-disease-5082930_final-75b1ce0a7bb1419595cf2b8b0773522c.jpg) The Role Of Nutrition In Sickle Cell Disease
The Role Of Nutrition In Sickle Cell Disease
 Sickle Cell Anemia Treatment Sickle Cell Anemia Treatment In India
Sickle Cell Anemia Treatment Sickle Cell Anemia Treatment In India

Comments
Post a Comment