Entri yang Diunggulkan
- Get link
- X
- Other Apps
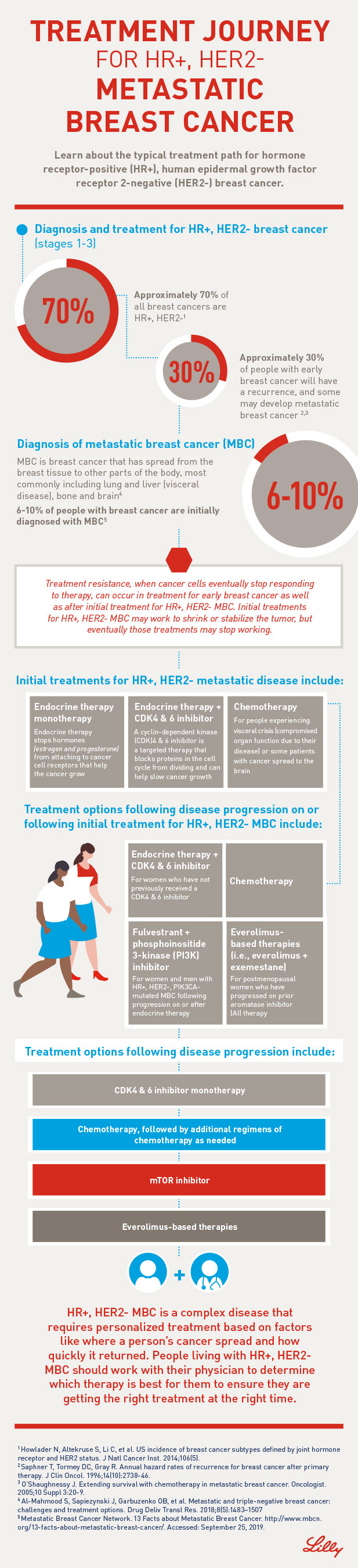
Across clinical trials MONARCH 1 MONARCH 2 MONARCH 3 33 of Verzenio-treated patients had ILDpneumonitis of any grade 06 had Grade 3 or 4 and 04 had fatal outcomes. Venous thromboembolic events were reported in 5 of patients treated with Verzenio plus fulvestrant in MONARCH 2 as compared to 09 of patients treated with fulvestrant plus placebo.
 Ndc 0002 6216 Verzenio Abemaciclib
Ndc 0002 6216 Verzenio Abemaciclib
Figures 2a and.

Verzenio patient reviews. The progression-free survival for patients taking VERZENIO with fulvestrant was about 16 months compared to 9 months for patients taking a placebo with fulvestrant. Verzenio abemaciclib is a brand-name drug that treats breast cancer. Eli Lilly received marketing approval from the FDA for Verzenio to be administered in combination with an endocrine therapy fulvestrant following endocrine therapy in September 2017.
It comes as a tablet. Verzenio Abemaciclib received an overall rating of 5 out of 10 stars from 3 reviews. Learn about side effects alternatives how it works and more.
Verzenio was administered at 200 mg orally every 12 hours on a continuous schedule until progression or unacceptable toxicity. The findings from the phase III MONARCH2 and 3 trials as well as the phase II MONARCH1 study led to these approvals. 11 hours agoIf a patient taking Verzenio discontinues a strong CYP3A inhibitor increase the Verzenio dose after 3 to 5 half-lives of the inhibitor to.
Action Date Submission Supplement Categories or Approval Type Letters Reviews Labels Patient Package Insert Note Url. Its still unclear how much longer the drug helps cancer patients survive than they would otherwise. See what others have said about Verzenio Abemaciclib including the effectiveness ease of use and side effects.
Verzenio abemaciclib was granted a priority review to a new drug application NDA to be used in combination with an aromatase inhibitor for the frontline treatment of women with hormone receptor HR-positive HER2-negative advanced or metastatic breast cancer according to Eli Lilly and Company the manufacturer of the drug. Abemaciclib Verzenio the only CDK46 inhibitor approved as a single-agent has amassed several clinical indications for patients with metastatic breast cancer. Find everything you need to know about Verzenio Abemaciclib including what it is used for warnings reviews side effects and interactions.
In clinical trials of Verzenio about 80 to 90 percent of patients experienced diarrhea. Proper use of Verzenio Medicines used to treat cancer are very strong and can have many side effects. Developed by Eli Lilly and Company Verzenio was granted priority review designation by the US Food and Drug Administration FDA in July 2017.
Before receiving this medicine make sure you. The most common side effect of Verzenio is diarrhea and for most people it begins during the first month of treatment. All three drugs treat HR-positive HER2-negative breast cancer.
Severe life-threatening or fatal interstitial lung disease ILD andor pneumonitis can occur in patients treated with Verzenio and other CDK46 inhibitors. Lilly didnt provide any details only noting that the benefit it observed is significant and that side effects were consistent with prior tests. Verzenio can also be used in a combination regimen but its the only one of the three that can be used on its own.
Verzenio abemaciclib is a small molecule drug and kinase inhibitor used to treat patients with advanced or metastatic breast cancer. Learn more about Verzenio Abemaciclib at. MONARCHe also studied Verzenio in HR-positive HER2-negative breast cancer.
For about 10 to 20 percent it was severe with several episodes of loose stools a day and difficulty controlling bowel movements. Matthew Goetz MD professor of oncology at the Mayo Clinic where he is also co-leader of the Womens Cancer Program and chair of the Breast Cancer Disease-Oriented Group discusses the research he presented at the 2018 San Antonio Breast Cancer Symposium on patient-reported outcomes from women being treated with Verzenio chemical name. With chemotherapy 689 percent 91 patients of patients had received a taxane and 553 percent 73 patients of patients had received capecitabine in the metastatic setting.
Verzenio is an ATP-competitive inhibitor of cyclin-dependent kinases 4 and 6 CDK 4 and CDK6.
Lilly Reports Improved Survival For Verzenio In P3 Trial Pharma 기사본문 Kbr
 Verzenio Abemaciclib Significantly Extends Life By A Median Of 9 4 Months For Women With Hr Her2 Advanced Breast Cancer In Monarch 2 Study
Verzenio Abemaciclib Significantly Extends Life By A Median Of 9 4 Months For Women With Hr Her2 Advanced Breast Cancer In Monarch 2 Study
Patient Stories Testimonials Verzenio Abemaciclib
 Novartis Kisqali Trailing Lilly S Verzenio Nabs Limited Nice Backing In Breast Cancer Fiercepharma
Novartis Kisqali Trailing Lilly S Verzenio Nabs Limited Nice Backing In Breast Cancer Fiercepharma
Patient Stories Testimonials Verzenio Abemaciclib
 Fda Grants Priority Review For Potential New Indication For Lilly S Verzenio Abemaciclib As Initial Treatment Of Advanced Breast Cancer
Fda Grants Priority Review For Potential New Indication For Lilly S Verzenio Abemaciclib As Initial Treatment Of Advanced Breast Cancer
 Buy Verzenio Verzenios Abemaciclib Price Costs Thesocialmedwork
Buy Verzenio Verzenios Abemaciclib Price Costs Thesocialmedwork
 How To Buy Verzenio Abemaciclib You Can Order Verzenio Abemaciclib Via 50 Best Pharma If The Drug Has Not Been Approved Or Is Not Available In The Patients Country 50bestpharma
How To Buy Verzenio Abemaciclib You Can Order Verzenio Abemaciclib Via 50 Best Pharma If The Drug Has Not Been Approved Or Is Not Available In The Patients Country 50bestpharma
Https Www Cadth Ca Sites Default Files Pcodr Reviews2019 10161abemaciclibmbc Inegr Noredact Abbrev Post 03may2019 Final Pdf
 Verzenio Abemaciclib Significantly Extends Life By A Median Of 9 4 Months For Women With Hr Her2 Advanced Breast Cancer In Monarch 2 Study
Verzenio Abemaciclib Significantly Extends Life By A Median Of 9 4 Months For Women With Hr Her2 Advanced Breast Cancer In Monarch 2 Study
 Patient Stories Testimonials Verzenio Abemaciclib
Patient Stories Testimonials Verzenio Abemaciclib
 Patient Stories Testimonials Verzenio Abemaciclib
Patient Stories Testimonials Verzenio Abemaciclib
Patient Stories Testimonials Verzenio Abemaciclib
Patient Stories Testimonials Verzenio Abemaciclib
Comments
Post a Comment